Lab Report: Synthesis Copper Hydroxide
- Petch
- Jan 4, 2019
- 1 min read
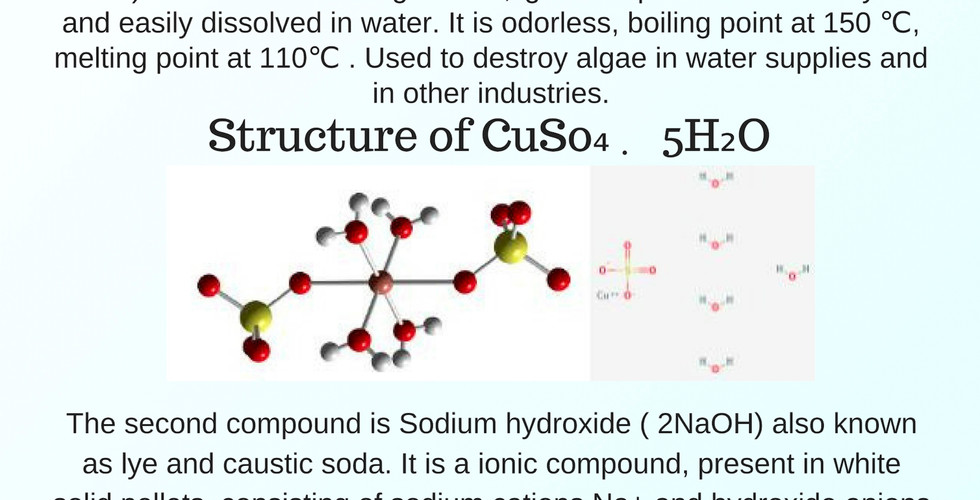
As a group of four, we do the experiment about Synthesis of Copper II Hydroxide. In this lab, we need to know how to calculate the mass relationship between both product and reagent to find the limiting reagent and excess regeant of the product. Then, calculating the percent yeild which is the result of our experiment.
This is our lab report which includes of different parts of the information, flowchart and result about the experiment. Moreover, it contains the discussion part that is provided our opinion, calculation and the errors of this lab.
The ESLOs that realted to this experiment are stratigic leaners because we used what we had learned in class and applying to lab .And another ESLO is articulates communicators because we did it as a group so we have to communicate with others to make the work successful.




































Comments